
“The NMPA (National Medical Products Administration of China) approved a total of 11,314 medical device registration items and the marketing of 35 innovative medical devices in 2021.” The NMPA has recently released policy interpretations related to the big data of medical device registration in 2021 and the list of innovative medical devices in 2021.
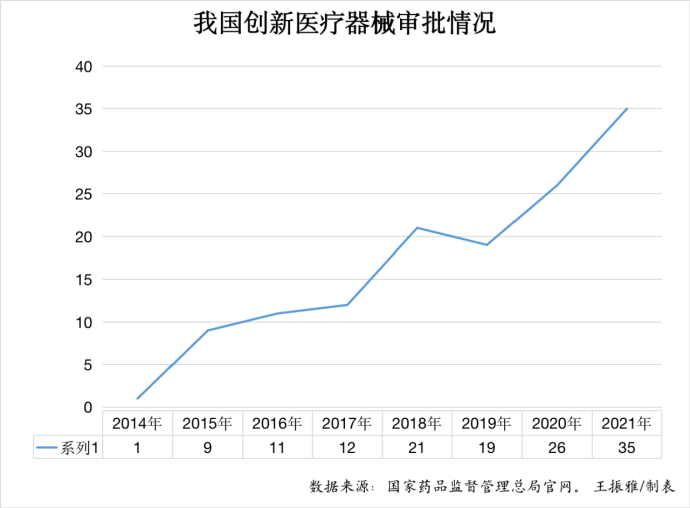
According to the reporter of the app of health.people.cn by sorting out related data, the number of innovative medical devices approved in China shows a rapid increase trend year by year from 1 in 2014, 26 in 2020, to 35 in 2021. The industry insider Xie Yijiong told the reporter of the app of health.people.cn that with 35 innovative medical devices marketed in a year, the number has hit a record high in China.
“Green channel” for medical devices
Innovative medical devices are medical devices that are granted with Chinese invention patents, feature technologies that are pioneering in China and leading in the world, and possess significant clinical application value. Such medical devices enjoy priority review and approval from the NMPA on the premise of not lowering the standards or simplifying the procedures. The green channel for innovative medical devices was opened in 2014.
The state drug regulator issued the Special Approval Procedure for Innovative Medical Devices (for Trial Implementation) in February 2014 (amended to Special Review Procedure for Innovative Medical Devices in 2018) to set up a fast track for innovative medical devices and encourage the R&D thereof. From the perspective of data, the number of innovative devices approved reached 1, 9, 11, 12, 21, 19, 26, and 35 respectively in 2014, 2015, 2016, 2017, 2018, 2019, 2020, and 2021.
The number of innovative medical devices approved for marketing has increased year by year, however, this figure is very small compared to the overall medical devices approval data, the reason for which is the high “threshold”, according to Xie Yijiong. By the end of 2021, there were a total of about 2,200 applications for special approval of innovative medical devices, with 134 products approved for marketing, accounting for only 5.2%.
Accelerated approval to promote the early marketing of medical devices
According to the statistics of the NMPA, the time required for products, which enter the special approval procedure for innovative medical devices, to obtain the registration certificates from the NMPA is average 83 days less than other products of the same kind. Xie Yijiong told the reporter that the early marketing of products thanks to the greatly shortened certificate obtainment period will undoubtedly increase product competitiveness and facilitate products to get a head start in the market.
For example, it took a total of nine months from application to certificate obtainment for MinFound’s ScintCare PET/CT, which was marvelously quick; it took a total of 13 months from application to certificate obtainment for Novel Medical’s SPECT, which was also well below the usual 24 months.
The Lifeflow™ Iliac Artery Bifurcation Stent Graft System independently developed by LifeTech Scientific Corporation was approved on January 13, 2021 as the first internal iliac artery revascularization device independently developed in China. It took 41 months from its entry into the “green channel” to approval.
Source: jksb.com.cn
© 2022 By YUSHI - All rights reserved.